The Ancient Earth 4. Where did all that water come from?


This post continues the series on the Ancient Earth and examines briefly the Origin of the Hydrosphere (oceans, rivers, lakes, ice-caps, groundwater).
The first few hundred million year
“Contemplate all this work of Time
The giant labouring in his youth“ (Tennyson ‘In Memoriam A.H.H.’)
Tennyson is perhaps referring to the immensity of Time, and a youthful earth beyond memory. But we do have a few numbers to contemplate.
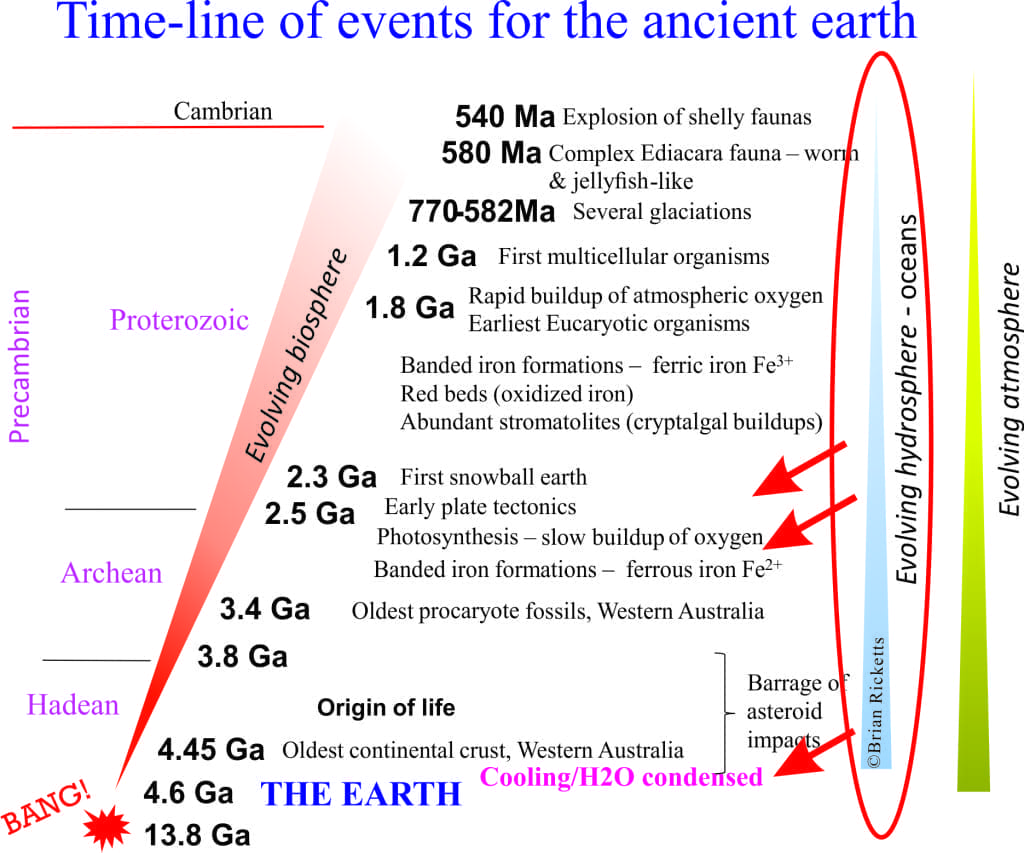
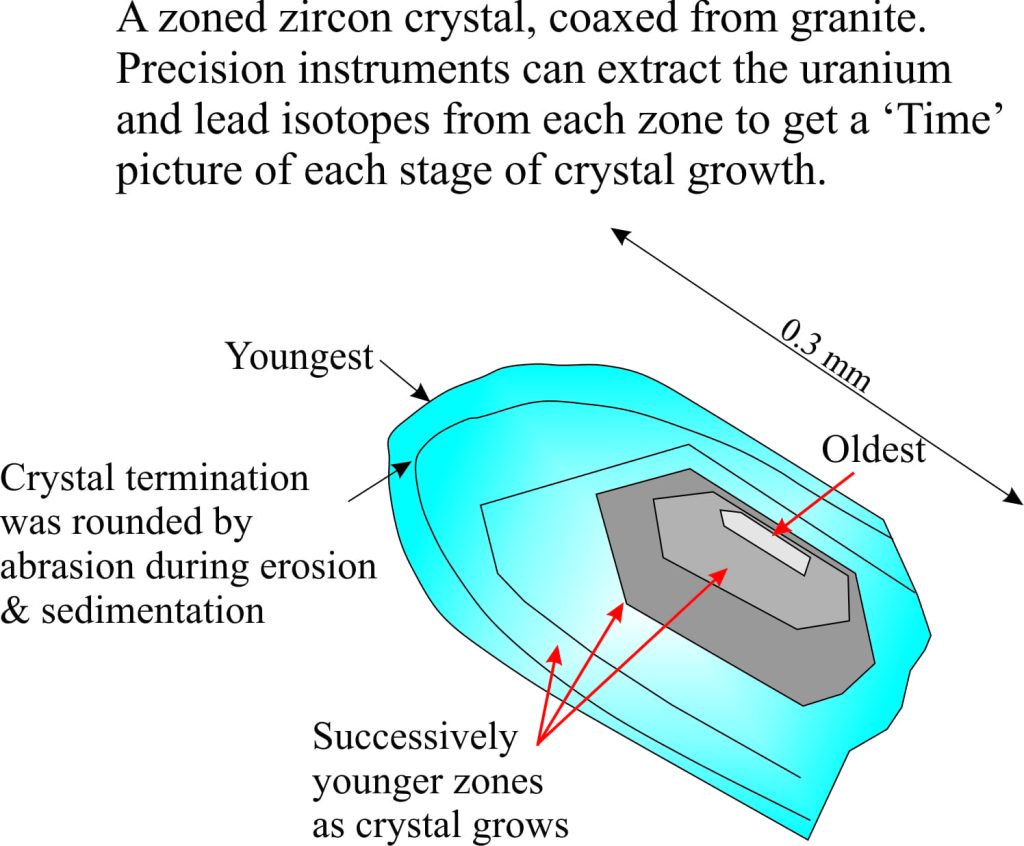
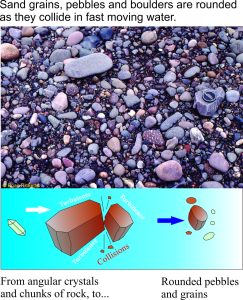
The earth is about 4600 million years old. The oldest solid earth or crust discovered so far is about 4400 million years old (based on the recovery of zircon crystals from Australia, which have been dated using radioactive isotopes). This in itself is important information, but for the present story a more critical piece of data comes from small parts of these zircons where the sharp crystal edges have been rounded by collisions with other grains or pebbles (called mechanical abrasion); these are dated at 4100 million years. In other words there is evidence that these zircon crystals were formed initially in granite, then removed from the granite by erosion and tumbled in fast moving water. If this is the case, then the earth had cooled sufficiently by 4.1 billion years for water to condense. Geologists now think that the oceans had begun to accumulate at this time. Thus, water vapour in the early atmosphere condensed, rain fell on the newly formed rocky surface, ran off in rivulets, streams and probably raging torrents to accumulate in depressions, or basins – the beginning of our seas and oceans.
More evidence of flowing water
In sedimentary rocks as old as 3400 million years we see very good evidence that there was flowing water on the earth’s surface. Structures like those shown below are common. In this example, ripples were formed in sand by tidal currents. Structures like these ripples are identical to those forming today (there are many different kinds of ripple-like structures).

In summary there is good evidence in ancient sedimentary rocks that water had accumulated before 3400 million years, possibly as early as 4100 million years ago.
Were the ancient oceans salty?
The average chemical composition of modern seawater is: Chloride (Cl) 55%, Sodium (Na) 30.6% Sulphate (SO4) 7.7% Magnesium (Mg) 3.7 Calcium (Ca) 1.2% Potasium (K) 1.1% bicarbonate (HCO3) 0.4%, and minor and trace components 0.3%. The saltiness derives from the combination of sodium and chloride to form the mineral Halite (NaCl). Na and Cl are two of the most abundant elements on earth.
We can determine the basic ingredients of ancient Precambrian seawater (although not their percentages) by examining the oldest sedimentary rocks (say from 3400 to 1800 million years ago) such as sandstone, limestone, 

All of the components of salty seawater were derived from the chemical breakdown of rocks, gases like CO2 and SO2 in the atmosphere, and the products of volcanic eruptions.
Salt accumulated in seawater because:
- Water vapour in the atmosphere and from volcanic gases condensed a few 100 million years after the earth formed.
- CO2 and perhaps some SO2, both soluble in water, acidified the rain.

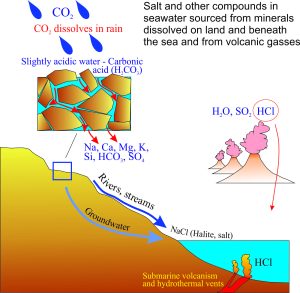
- Acid rain percolated through fractures and pore spaces in rocks near the earth’s surface.
- Minerals that made up the rocks began to dissolve.
- The different metal elements and other compounds eventually flowed into the accumulating bodies of water.
- Volcanic eruptions also produced small amounts of hydrochloric acid (HCl), that over time provided the chlorine that combined with sodium to form salt (NaCl).
Layered oceans
Modern oceans are stratified, or layered. Layering is caused by several factors, such as density, where cold heavy water lies beneath warmer lighter water. Oxygen levels can also change with depth, where turbulence mixes air and water in shallow waters. We also know that salinity varies both geographically and with depth.
Geologists now think that the ancient oceans were also stratified. For example, prior to about 2500 million years ago when oxygen levels began to increase in the atmosphere, there was little or no oxygen in the oceans. In oceans older than 2500 million years the reduced form of iron (Fe²+) would have been stable. In younger oceans that began to accumulate oxygen, there may have been deep layers where the reduced form of iron (Fe²+) was stable, possibly because of high hydrogen sulphide conccentrations (rotten egg smell), and shallower waters with higher levels of oxygen where dissolved iron was oxidized, resulting in the characteristic rust-coloured iron minerals that were deposited as banded iron formations.
Foot note
Each post on the origins of oceans, air and life, tend to each sphere separately. This is a convenience only. I cannot stress enough the importance that the formation and evolution of each sphere (atmosphere, hydrosphere and biosphere) was inextricably linked to each other sphere. Science can hypothesize about many of the loops, feed-backs, one-way, and even dead-end chemical and physical systems that must have operated. There is no question that science is only beginning to understand the level of complexity of these really ancient processes.