The image above illustrates part of a familiar grand cycle; water evaporated from the oceans, precipitates, is gathered in rivers and lakes that eventually return it from whence it came. This accounts for about 2% of earth’s available fresh water. The other 98% resides underground in aquifers. Groundwater is part of this grand cycle, albeit a less familiar part.
Almost half the world’s population lives within 60km of a coast; 75% of large cities are located near coasts. Coastal aquifers are part, in many cases the only part of the water supply equation to these crowds. Unfortunately, as is the case with so many of our water supplies, we have (collectively) failed to look after these resources.
Aquifers don’t just stop at the shoreline. Both unconfined (watertable) and confined aquifers extend beneath the sea floor, commonly many 10s of kilometres across continental shelves. There is a dynamic relationship between the land-derived fresh water, and sea water that enters the aquifer beyond the coast. Herein lies the problem; pumping of coastal aquifers without due diligence results in the all-too familiar saline, or sea water intrusion.
Aquifers at the coast
The cartoon below illustrates the interaction between fresh and sea water in coastal aquifers.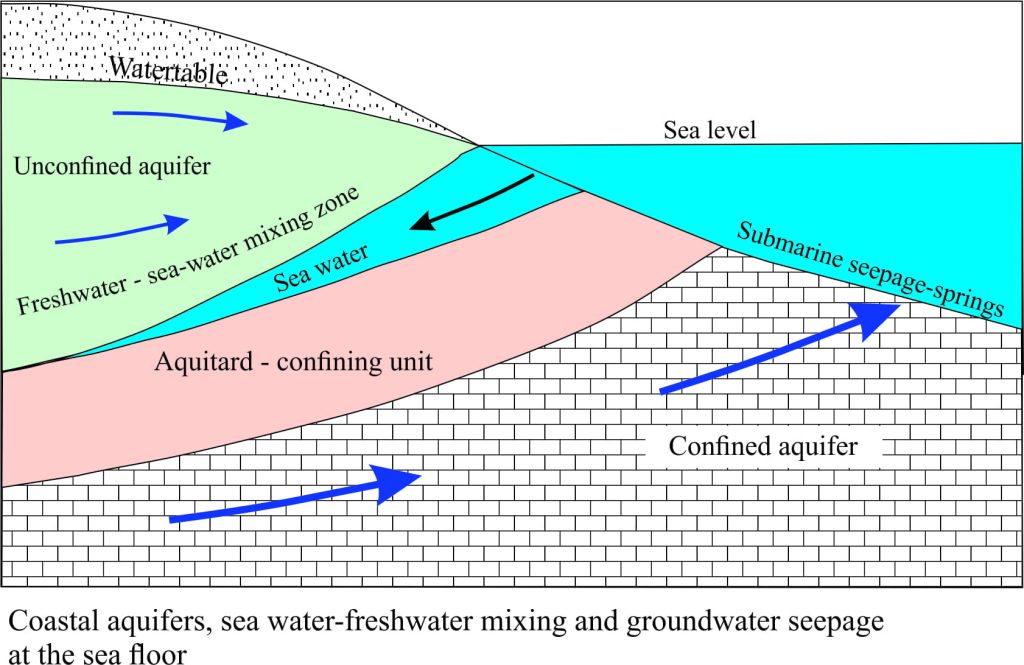

Groundwater at sea
Fresh water seepage at the sea floor is a common phenomenon although it tends to be highly variable from one place to another. Evidence for this is seen as pockmarks on the sea floor, springs, or where sea water is locally diluted. Submarine groundwater seepage is also implicated in some slope failures (submarine landslides); this has important implications for constructions that extend across the sea floor. Groundwater seepage reduces the shear strength of sediment to the point where failure and sediment sliding or flow takes place.
An important but negative effect of submarine seepage occurs where groundwater contaminants enter the marine food chain in estuaries and shallow seas. Nitrates and phosphates in particular are known to exacerbate marine algal blooms (this also occurs in lakes).
Fisherman, from the islands of Bahrain and Arad (southern Persian Gulf) have for centuries found fresh water by diving to the sea floor and filling bags with water gushing from submarine springs. The submarine springs issue from limestone aquifers that are recharged on the mainland. Unfortunately these springs are threatened by saline intrusion caused by over-pumping of land-based wells.
The Floridan Aquifer is another classic example of a groundwater system extending far offshore. Floridan (Tertiary) limestone and dolomite aquifers are utilized in several states. Exploratory drilling on the offshore platform in 1965 discovered fresh and brackish water in boreholes up to 120km offshore and 130m below the sea floor. In one borehole fresh water flowed 2m above the ship’s deck. (Source: Manheim, F.T. 1967 Evidence for submarine discharge of water on the Atlantic continental slope of the southern United States, and suggestions for further research New York Academy of Sciences Transactions, Series 2, v.29, p. 839-853).
A great example from Yucatan Peninsula was kindly provided by Nigel Platt – © Nigel Platt, Edison E&P UK Ltd. The fresh water springs across this shallow platform at Casa Cenote, Tankah Beach, approximately 10 km N of Tulúm in Quintana Roo, upwell through the platform carbonates. Freshwater is recharged from the highlands a few 100 km inland. Flow is focused through a complex network of limestone caves to the coast. The fresh water has a significant impact on the adjacent platform, its biota, the production of carbonate sediment and sea floor cementation.
Some fresh water beneath the continental shelf may be relict, and not necessarily connected to present avenues for recharge. An example of this might be groundwater that accumulated when the shelf was exposed during the Last Glacial low sea-level, and subsequently was stranded as sea level rose.
A recent paper in the journal Nature posits that the volume of fresh and low salinity water beneath continental shelves around the world could be 500,000 km3, most of it at shallow depths beneath the sea floor. Much of this water is effectively non-renewable, at least in terms of recharge rates that could keep pace with potential extraction.
Sea Water Intrusion
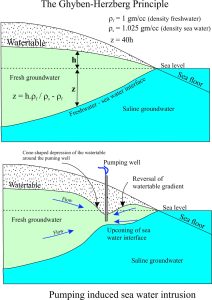
The sea water – freshwater interface in an unconfined coastal aquifer is not an abrupt boundary, but a diffuse zone where mixing of the two waters takes place. If we know the depth to the watertable (usually from drilling or modelling), we can calculate the depth to the interface. The calculation, shown in the diagram, assumes the interface is an abrupt boundary. Despite this simplification borehole drilling has shown it to be a good approximation of the interface depth. It is known as the Ghyben-Herzberg Principle and is based on the ratio of freshwater/sea water densities. Basically the principle states that for every unit depth (m, ft) of freshwater above sea level (‘h’ in the diagram), there will be 40 depth units (m, ft) below sea level (‘z’).
The Ghyben-Herzberg Principle is useful because it helps us to gauge the potential for groundwater pumping to distort the freshwater-sea water interface. An important corollary of the principle states that for every unit depth (m, ft) drop in the watertable, there will be a corresponding 40 unit depth rise in the interface. Again, this relationship has been verified many times by drilling. Clearly, it doesn’t take much of a drop in the watertable to produce a drastic change in the potential for sea water intrusion. Sea water intrusion into coastal aquifers is for all practical purposes, non-reversible.
Examples where this is occurring are numerous:
- Gaza Strip,
- Florida and other parts of the Floridan Aquifer, Dade County,
- coastal aquifers that supply Jakarta
- Bahrain, Indonesia and Bangladesh, – to name a few of the coastal aquifers that are critical for the welfare of many millions of people.
Sea level rise will exacerbate the problem because the sea water-freshwater interface will tend to move upward and landward.
Acceptable salinity levels in potable water vary from country to country. Values above 500mg/L are generally considered poor; the UN recommends a maximum 250mg/L (sea water averages 35,000mg/L). The problems with elevated salinity are not just about taste; it is also one of the major causes of renal failure. Irrigation with water containing elevated salinity not only effects crops, but over time (with evaporation) creates saline soils that ultimately will fail to support cropping.


Pingback: click here