Some basic geochemistry of carbonates, carbonic acid and carbon dioxide
This is part of the of How To…series… on carbonate rocks
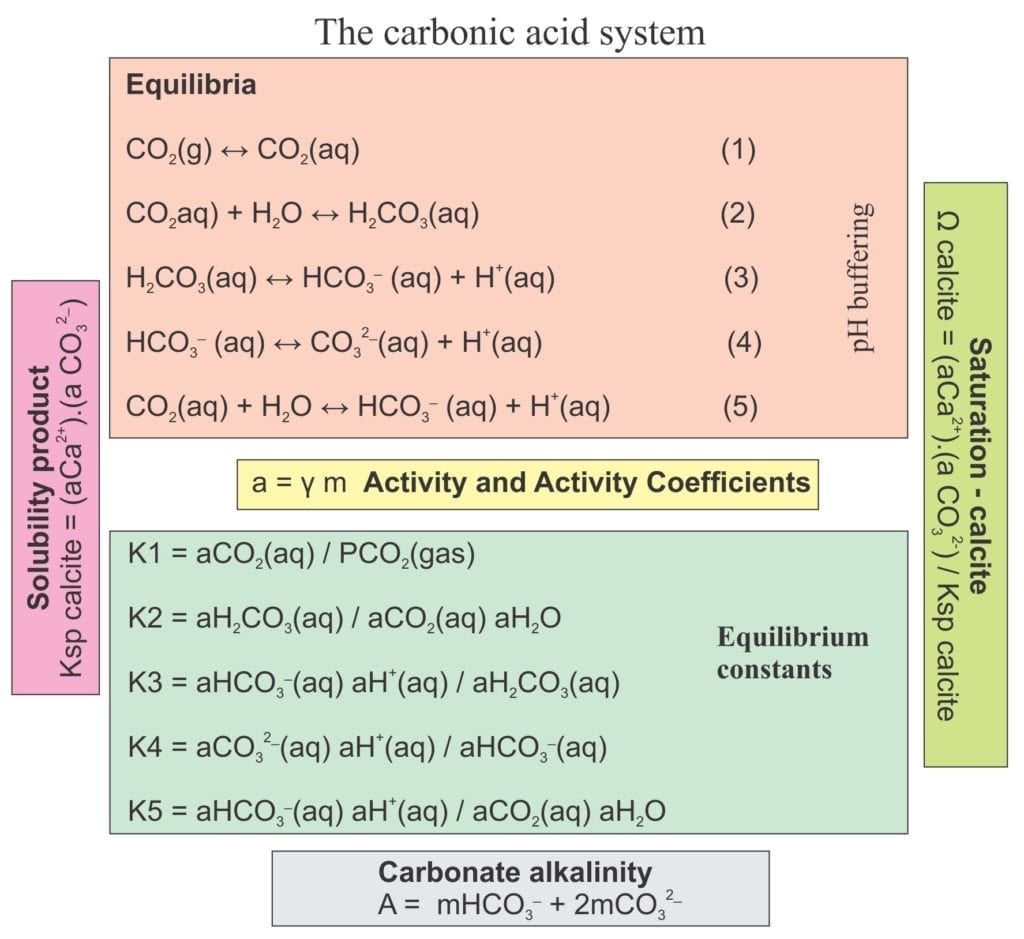
Sedimentary carbonate petrology is concerned first and foremost with the precipitation and dissolution of mineral phases; principally calcite, aragonite and dolomite. Both processes involve chemical reactions and the two primary requirements for these reactions are:
- thermodynamics – there needs to be sufficient energy to drive the reactions, and
- an excess or deficit of dissolved mass that, for the minerals of interest, includes Ca2+(aq), Mg2+(aq), and CO32-(aq) (aq = aqueous)
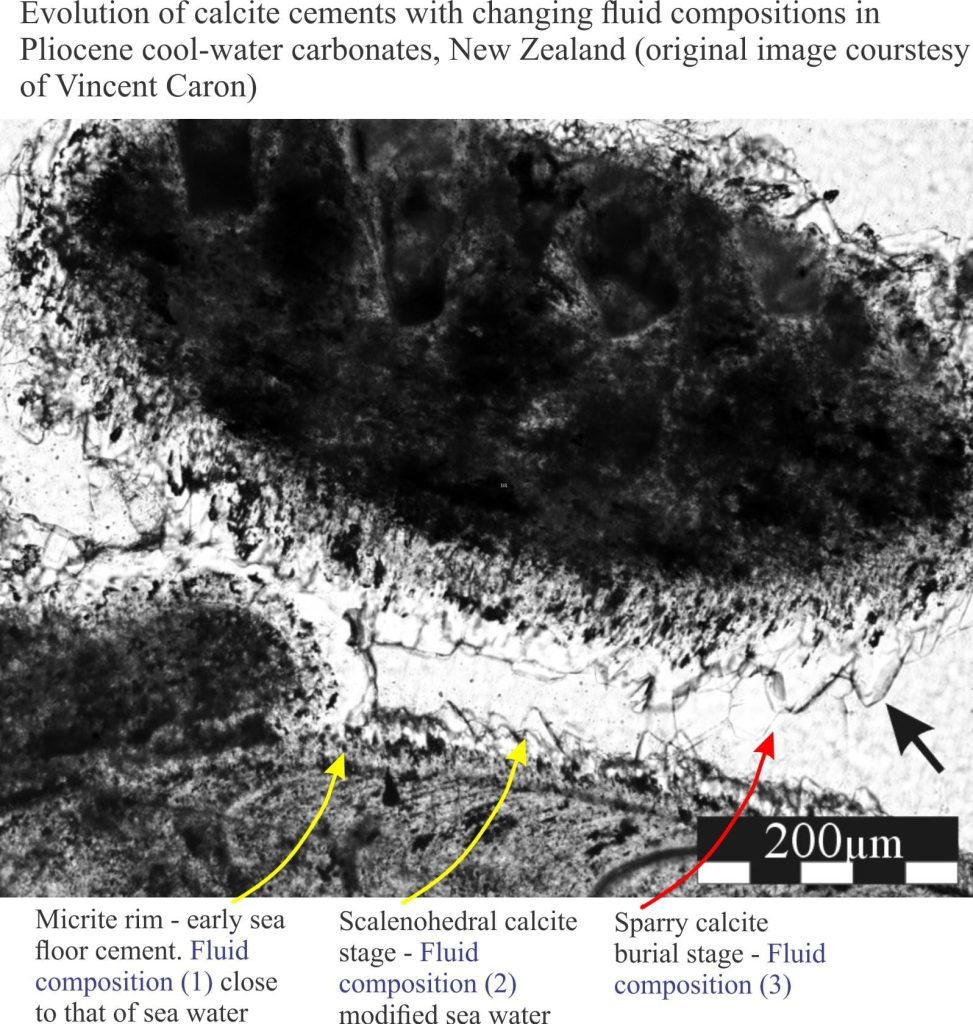
All sedimentary carbonate reactions at the surface or during sediment burial take place in water: fresh water, sea water, or concentrated brines. Furthermore, the chemical composition of these fluids can evolve, for example during burial (sea water to brine), or uplift and exposure (sea water to fresh meteoric water). Such changes are commonly manifested as cement stratigraphy (e.g. high-Mg calcite overlain by low-Mg calcite), or where clasts, matrix and early cements are replaced by new generations of calcite or dolomite.
We are also interested in carbonate reactions because of the present trend towards climate change and the possibility that ocean chemistry may change; ocean acidification tops the list here. To get beyond the hyperbole, to determine whether this is a real possibility or not, we need to understand some basic chemistry. Some introductory concepts are outlined herein.
To delve deeper into the complexities of the carbonate system, have a read of the contributions cited below. Continue reading