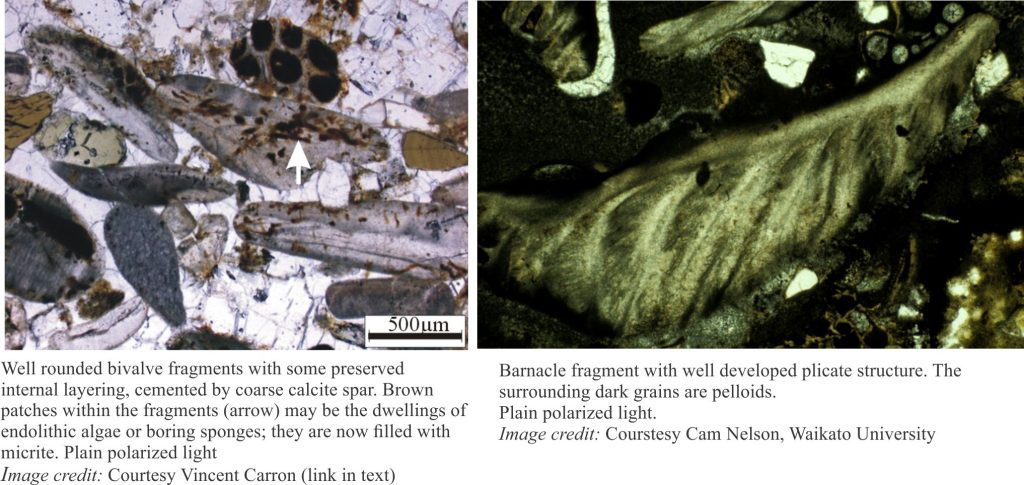
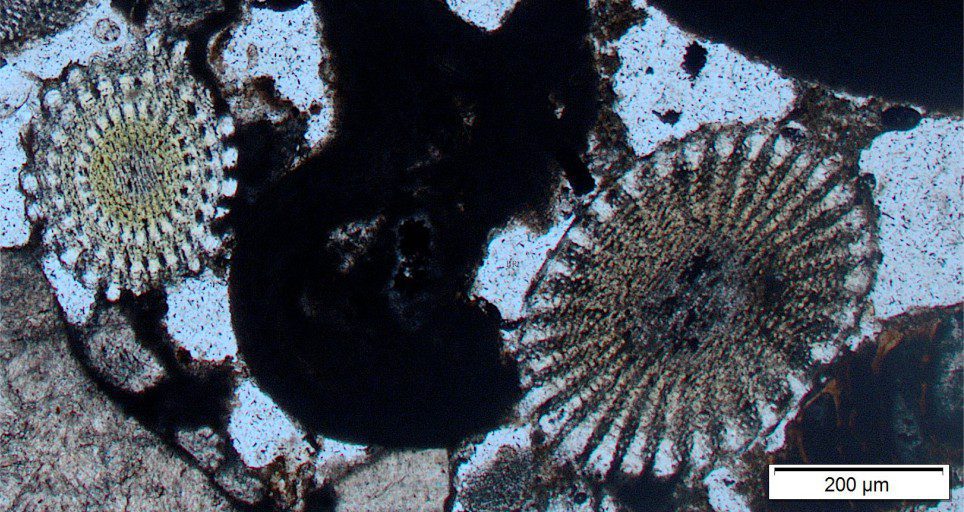
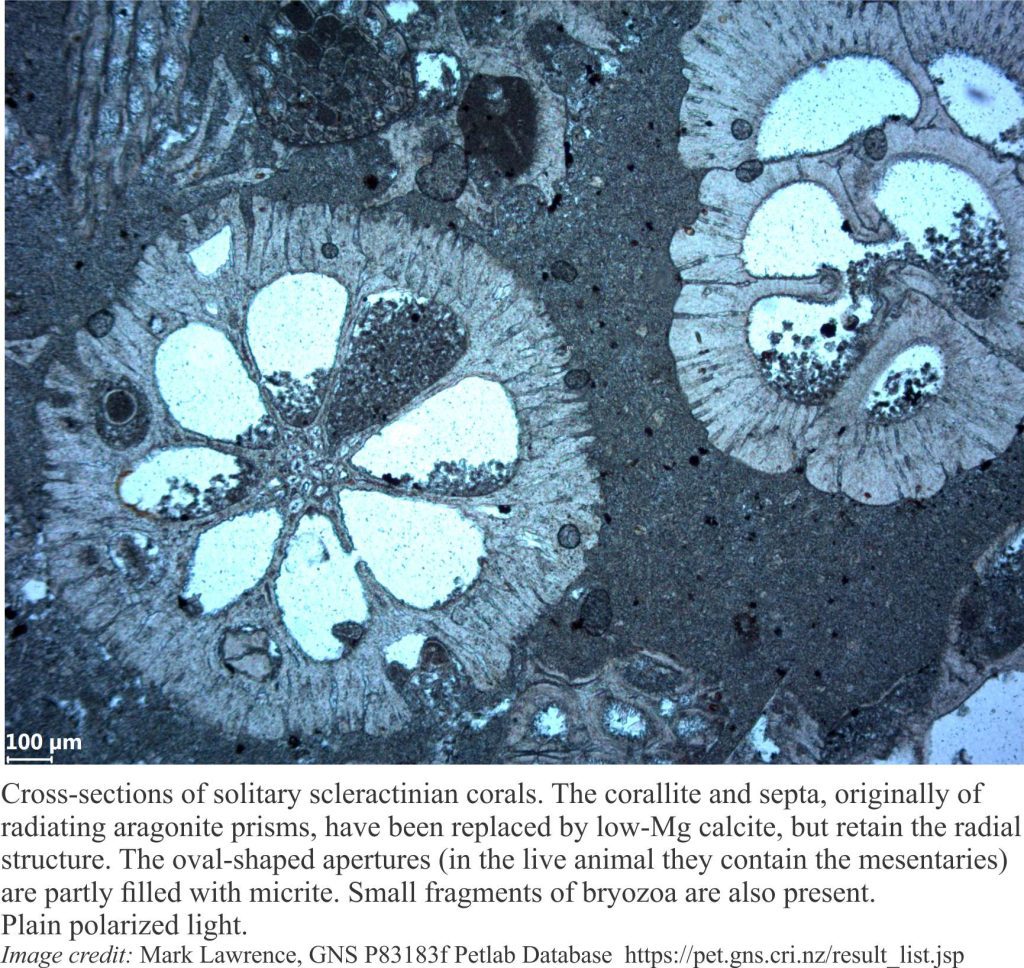
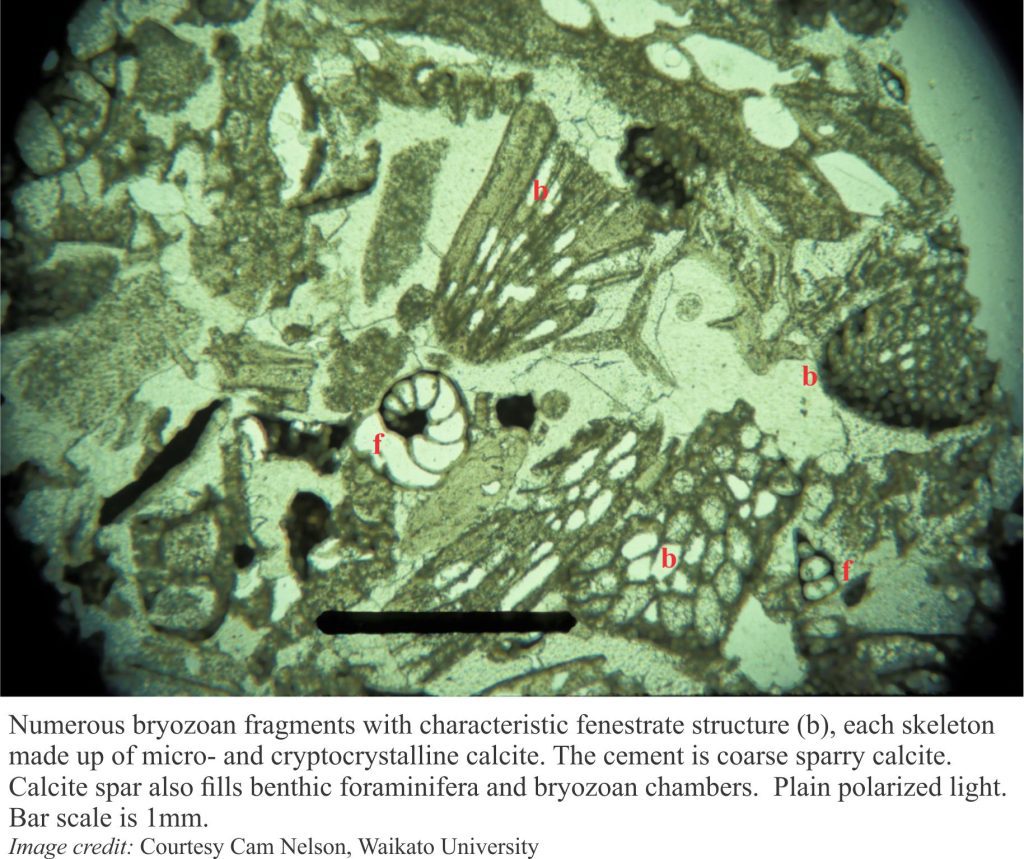
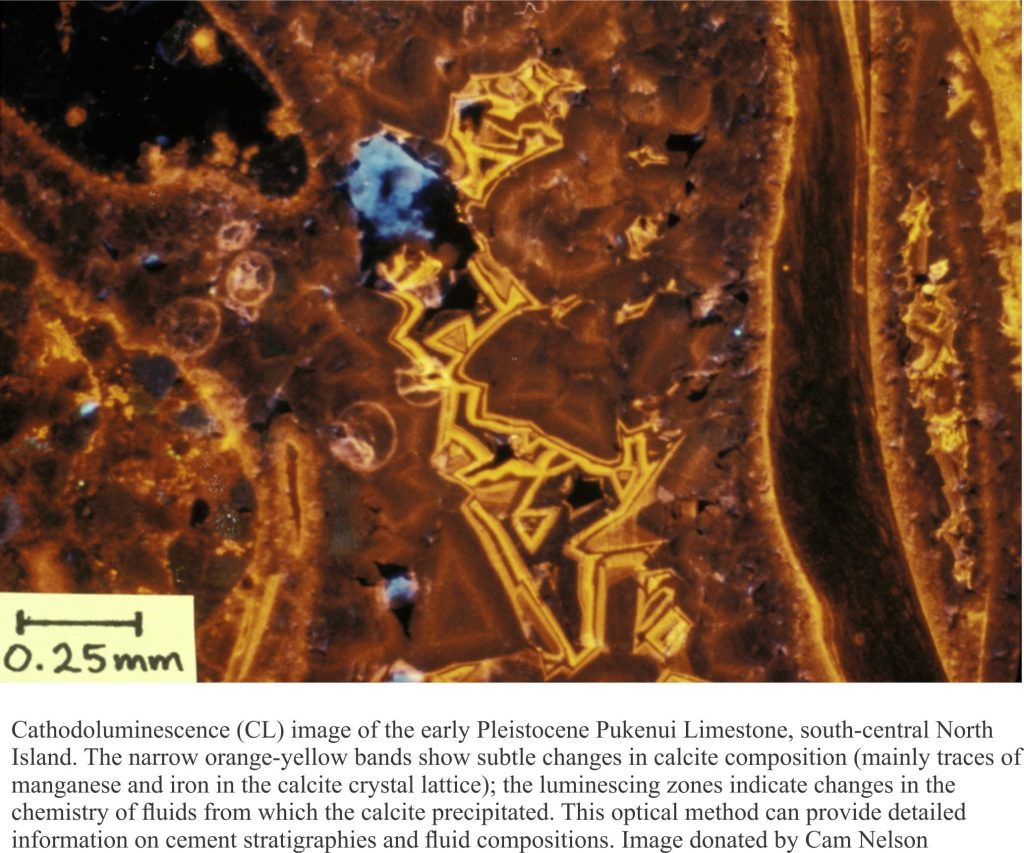
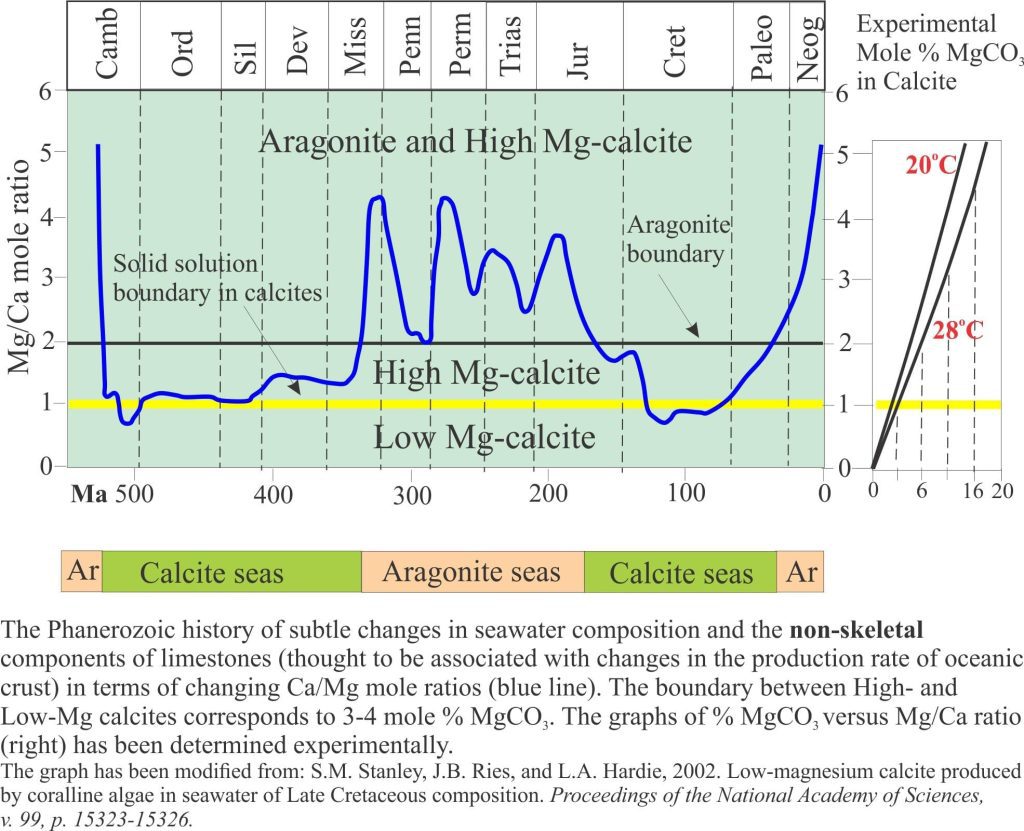
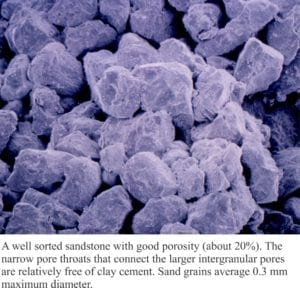
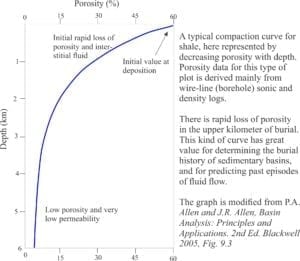
Carbonate diagenesis; How limestones form.
This is part of the of How To…series… on carbonate rocks
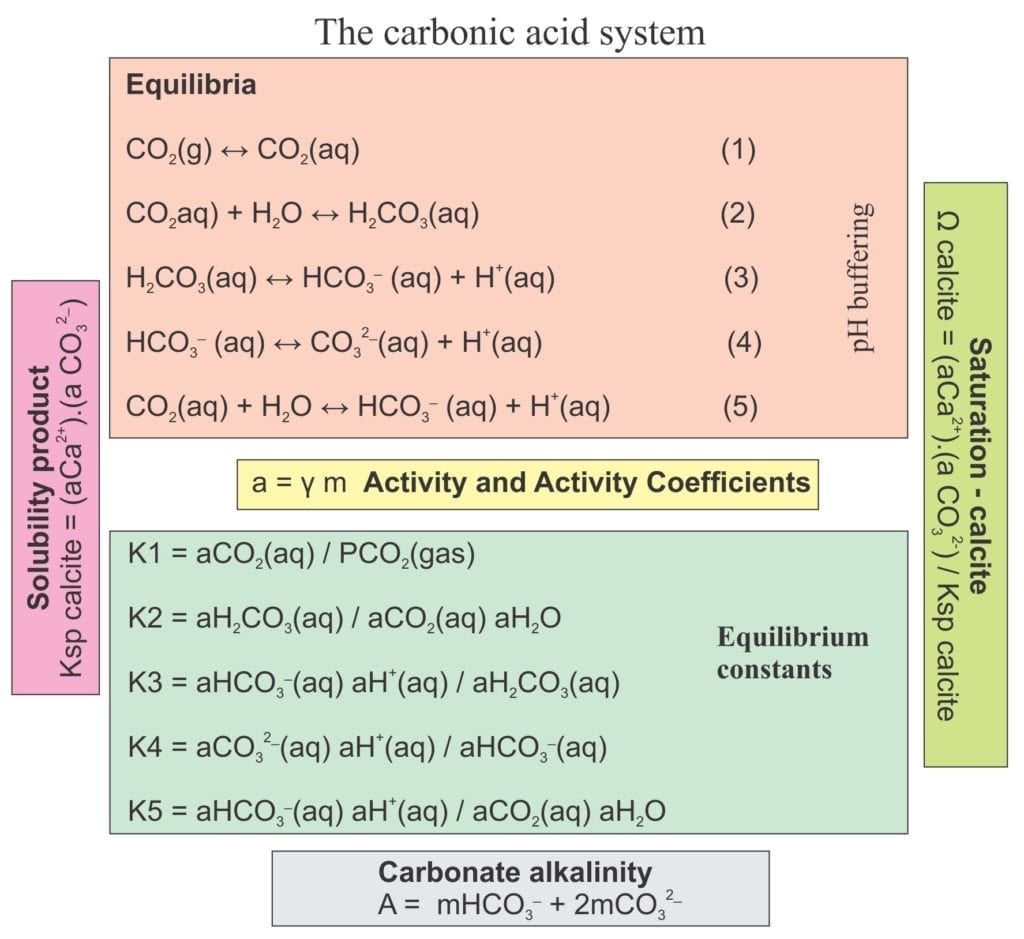
Of all the common rock-forming minerals, carbonates are the most reactive chemically. The transformation of loose sediment to hard limestone involves chemical reactions that, depending on the conditions (ionic concentrations, pH, degree of saturation, temperature) promote precipitation or dissolution of minerals, most commonly calcite, Mg-calcite, aragonite and dolomite. These reactions take place at the surface (e.g. sea floor) and at all stages during sediment burial and uplift. Limestone diagenetic pathways are complicated; this is part of the attraction for those who study them (notwithstanding the opportunity to conduct field work in places like Bahamas).
Some general requirements for diagenesis to proceed are:
- The thermodynamic stability and metastability of precipitating phases are determined by pressure, temperature and chemical composition of the fluids (including partial CO2 pressures, pH, and Mg/Ca ratios).
- Reactions take place in water: sea water, modified sea water, fresh water and saline brines. These fluids are never static; they flow, delivering new solute (ions in solution) to sites of precipitation, and removing dissolved solids to other sites in the permeable sediment.
- Carbonate diagenesis at all burial depths is, like any other rock type, governed by subsurface fluid flow and evolving fluid compositions. Fluid flow itself is governed by hydraulic gradients that are generated by topography, sediment compaction and tectonic loads.
- If the conditions change, any phase that becomes thermodynamically unstable will dissolve. This applies particularly to metastable phases like aragonite and high Mg-calcite, where changes in the fluid environment can render them unstable and prone to dissolution. Fluid composition will change when, for example, shallow sea floor carbonates are exposed to meteoric fresh water as sea level falls, or during burial where original seawater is modified by reactions involving carbonates, siliciclastics and, importantly organic matter.
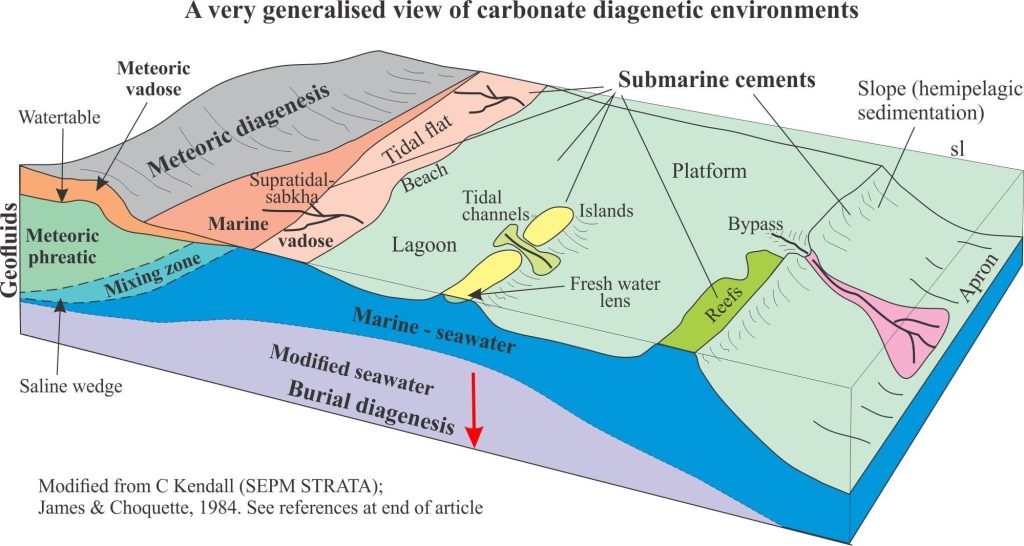
Geologists like to subdivide things and the carbonate diagenetic realm is no different (it helps to simplify a complex world). Three diagenetic environments are frequently cited, with a fourth located at the transition to metamorphism (each environment will be treated in greater detail in separate articles), and depicted in the diagram below:
- Seafloor environments (almost syndepositional), including the first few centimetres or metres of burial.
- Shallow burial – meteoric environments
- Deep burial environments
Sea floor diagenesis
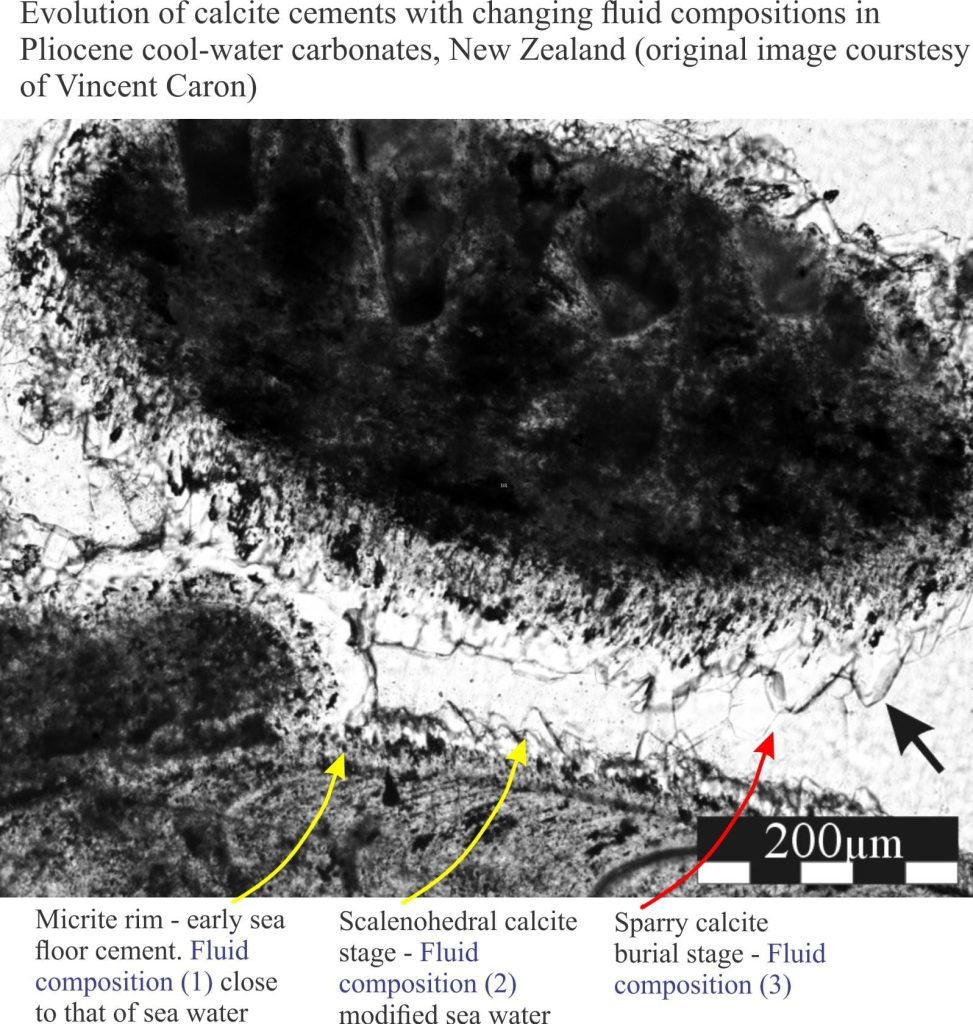
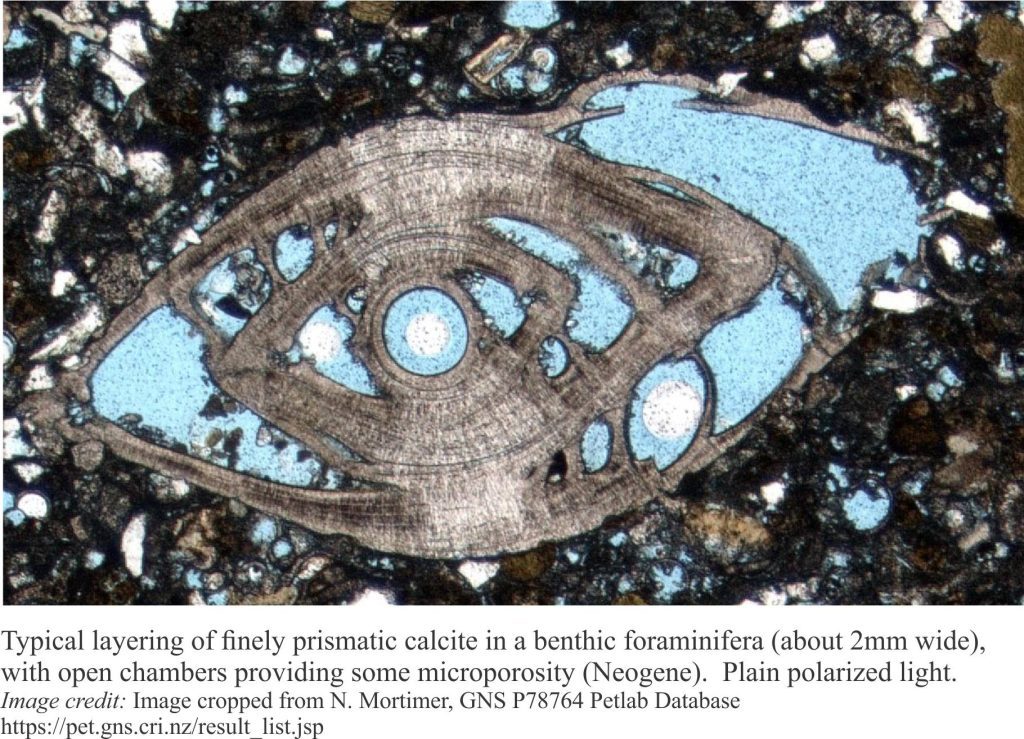
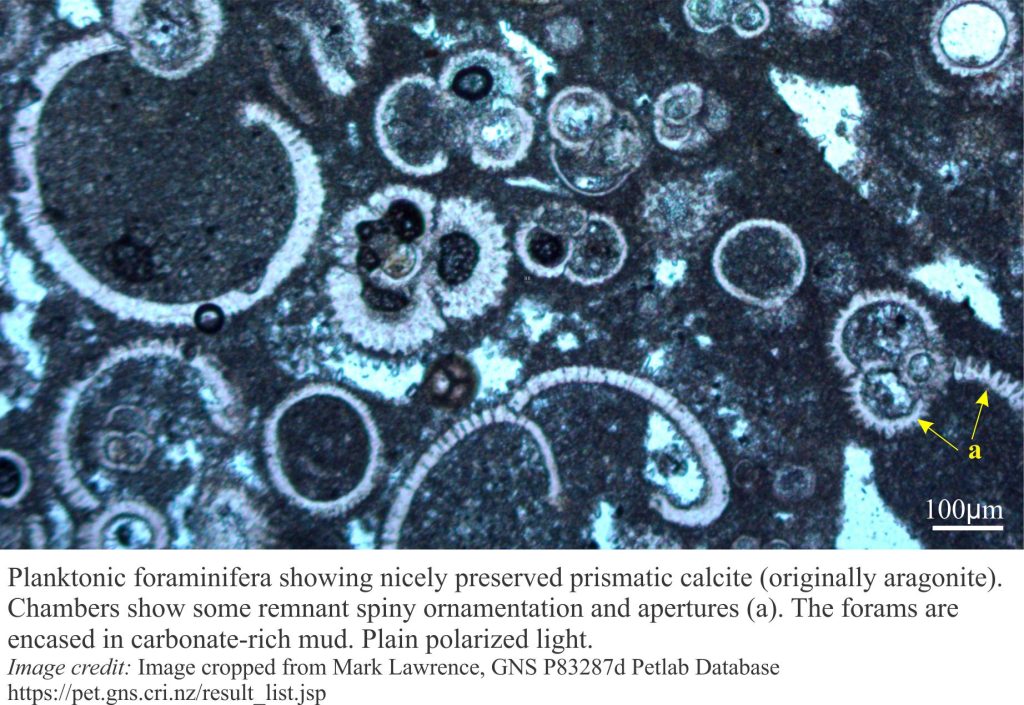
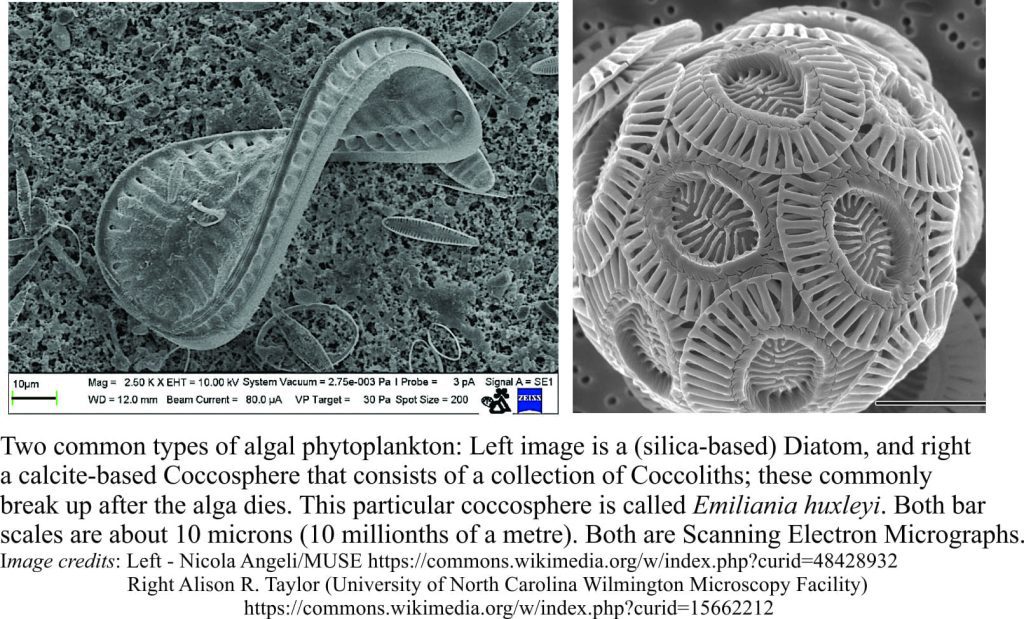
Precipitation of aragonite and high-Mg calcite cements on the sea floor or in the first few centimetres to metres beneath it, is common in tropical settings, less so in cooler waters. It is the region influenced by ambient seawater compositions; it is also referred to as the marine phreatic zone.
This, the earliest stage of carbonate diagenesis is promoted by low sedimentation rates, high levels of mineral supersaturation in seawater and rapid exchange of atmospheric CO2 with aerated seawater. Microorganisms like bacteria and photosynthesizing algae also contribute, either as mediators or by direct precipitation. Some microorganisms such as endolithic bacteria and algae have a dual role in that their substrate boring activities tend to destroy primary grains but leave micrite rinds and infills in their wake.
Seafloor cementation can take place from the supratidal-intertidal zone (marine vadose zone) (e.g. crusts, beachrock), reef and shallow subtidal platform (mainly within the photic zone), to the outer platform and slope (beyond the photic zone). At greater depths, first aragonite (the aragonite compensation depth – ACD – is 2-3km) then calcite (CCD is 4-6km) begin to dissolve because of high CO2 partial pressures.
Meteoric environments
Platform and reef deposits exposed by a fall in relative sea level will be subjected to an influx of fresh water. Carbonate deposits subjected to meteoric conditions undergo significant changes to their mineralogy and texture.
In the meteoric environment, groundwater flow through permeable aquifers is driven largely by gravitational potential energy, usually referred to as topography-driven flow. Hydrogeologists have long recognized three distinct zones within meteoric settings; James and Choquette (reference at bottom of the page) included these zones in their early model of carbonate diagenesis:
- The watertable, below which all porosity is completely saturated; this is the phreatic zone.
- Above the watertable is the unsaturated or vadose zone where pore spaces are mostly air-filled but are periodically wetted by rising watertables (may be seasonal) and infiltration of surface water.
- Where aquifers intersect the coast there is a zone of fresh water and seawater mixing. The location of the phreatic mixing zone in relation to the shoreline depends on the hydraulic gradient (or hydraulic head) in the aquifer and how far the aquifer extends offshore. It is not uncommon for fresh water to flow 100s, even 1000s of metres offshore, beneath a platform or shelf.
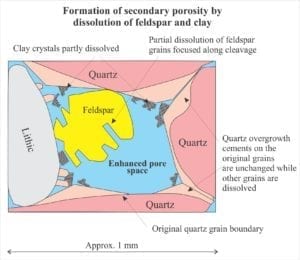
Aragonite and high-Mg calcite are unstable in fresh water which means that any clasts (skeletal fragments, ooids, foram tests) and cements that contain these minerals will begin to dissolve. In some cases, secondary porosity will form. Also common is the replacement of clasts and cements by low-Mg calcite.
Karst landscapes form during prolonged exposure to meteoric conditions where even the low-Mg calcites dissolve.
Deep burial environments
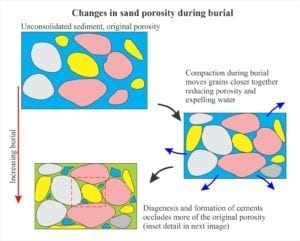
Deep burial usually refers to the interval below the influence of marine phreatic and meteoric fluids (10s to 100s of metres deep) to several 1000m depth (depending on the geothermal gradient). The principle physical process is compaction that rearranges sediment frameworks and drives interstitial fluids to other parts of the sedimentary basin. The influence of temperature on the promotion and rates of chemical reactions becomes increasingly important with depth.
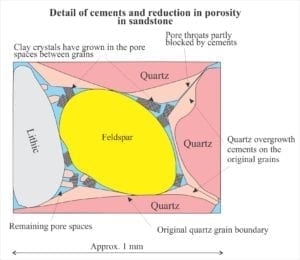
Fluid composition is primarily modified seawater, modification that can eventually produce saline brines. Many different reactions come to play as burial depth and temperature increase. Compaction enhances pressure solution where significant volumes of rock are dissolved and the solute transferred to other parts of the sedimentary basin; what remains are stylolites. Other reactions involve dehydration of clays, clay mineral transformations (e.g. kaolinite-smectite), and perhaps most significantly, the diagenesis of organic matter. All these reactions contribute to changes in pH and alkalinity that effect calcite and dolomite stability.
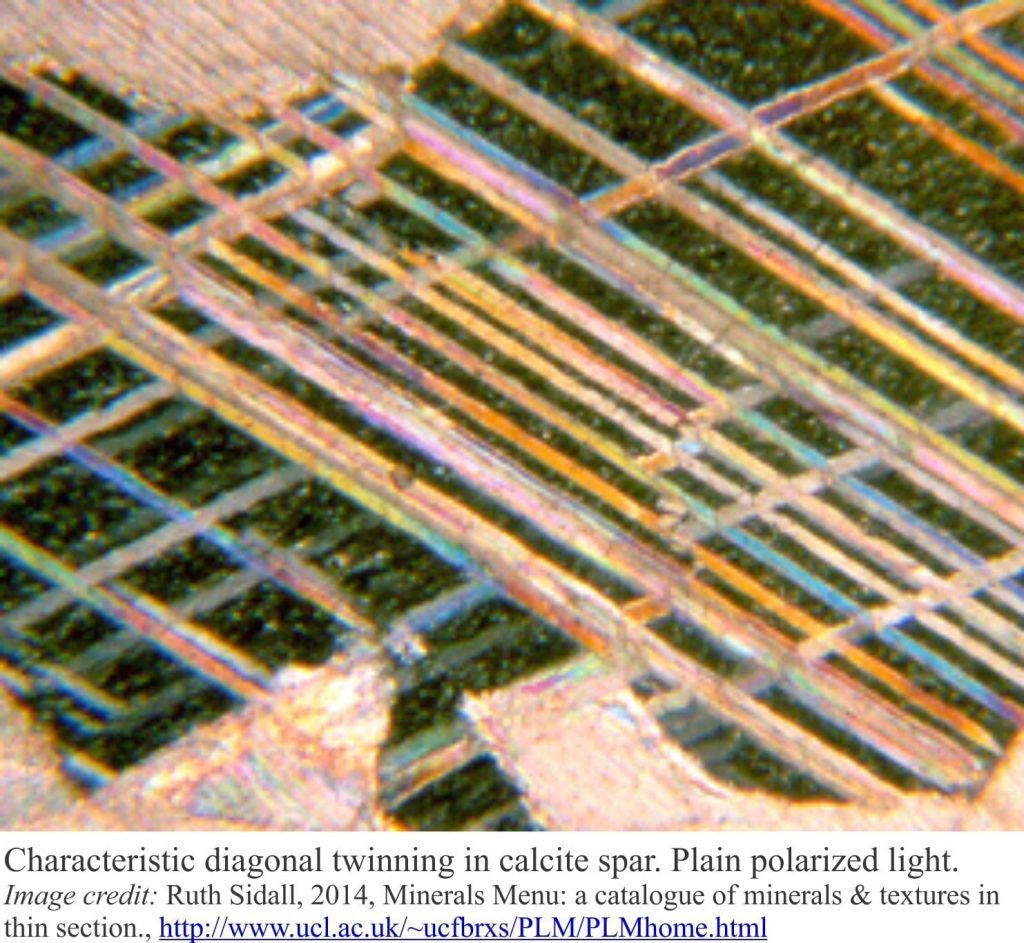
Carbonate diagenesis is dominated by precipitation of calcite and ferroan calcite that replace aragonite and high-Mg calcite. Recrystallization of calcite spar tends to mask and even obliterate original depositional fabrics. Dolomitization is also common during burial diagenesis and like calcite overprints earlier grain and cement fabrics (i.e. those formed in the sea floor and meteoric environments.
Companion diagenetic environments
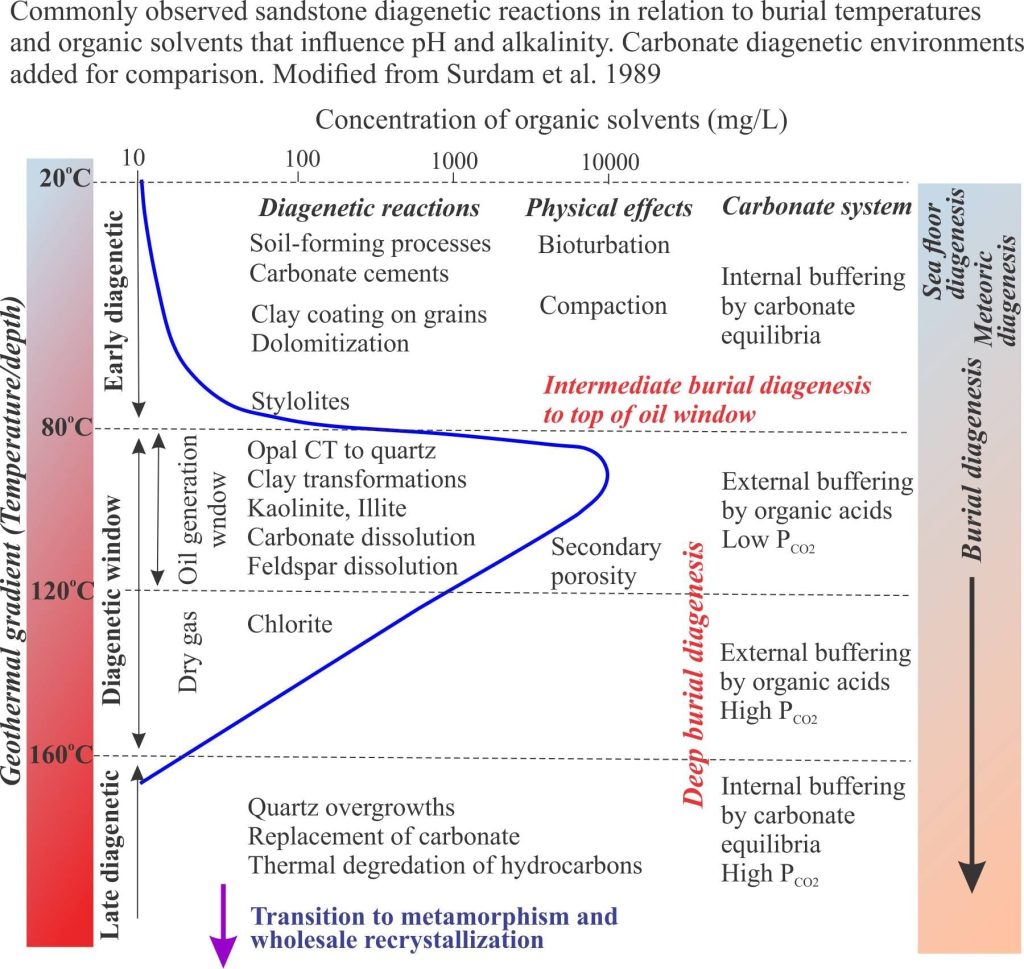
It is important to recognize that the diagenesis of carbonates during burial is not divorced from broadly similar processes taking place in siliciclastic rocks. Precipitation of quartz and clays, and dissolution of feldspar are important determinants for evolving fluid compositions that significantly effect carbonate mineral stability (mostly low Mg-calcite). Organic matter in carbonates and siliciclastics has a profound effect on deep burial diagenetic pathways. Complex organic compounds like kerogens begin to break down at about 60o C. The rate of organic diagenesis increases markedly at 80o C – the lower burial temperature limit of the oil-generation window. Important byproducts of these reactions are organic acids that modify pH and control alkalinity. Note too that pH buffering and alkalinity are also influenced by silicate transformations, particularly those involving smectite, kaolinite and illite. The diagram above (from Surdam and others, 1989) summarizes the progression of diagenetic reactions commonly observed in siliciclastic rocks, in relation to organic maturation, organic acids and pH buffering.
Links to other posts in this series:
Mineralogy of carbonates; skeletal grains
Mineralogy of carbonates; non-skeletal grains
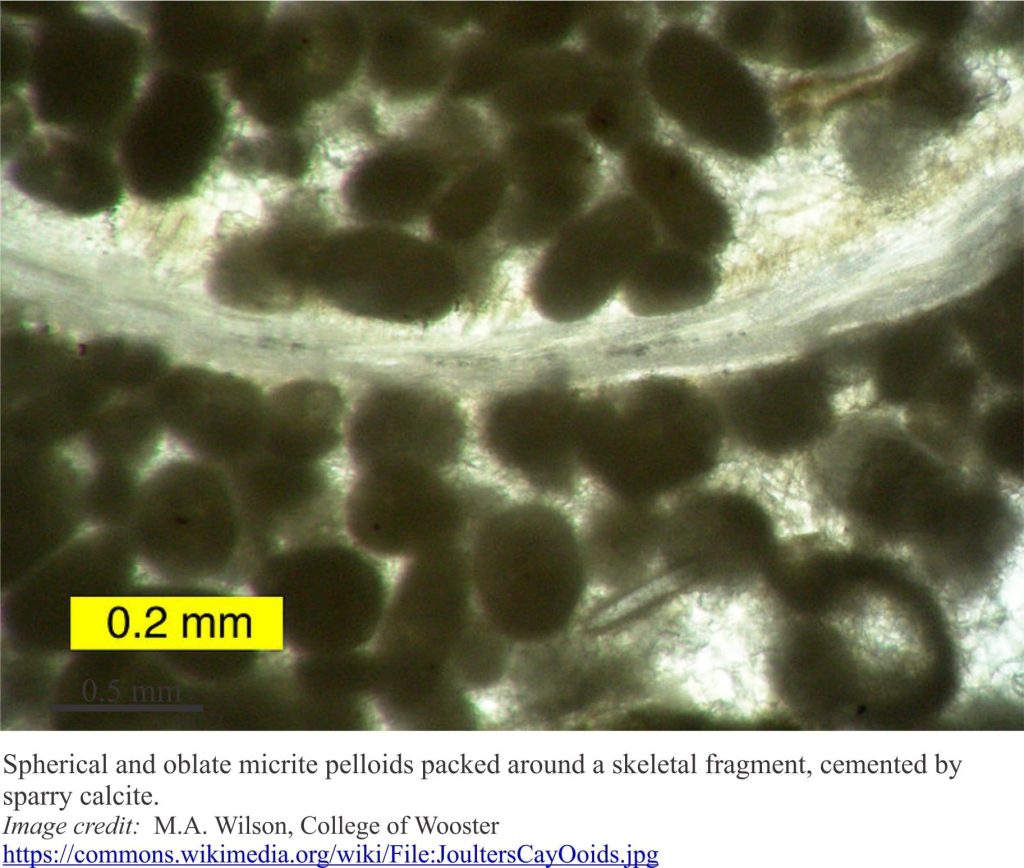
Mineralogy of carbonates; lime mud
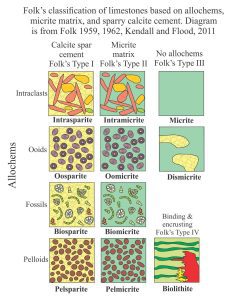
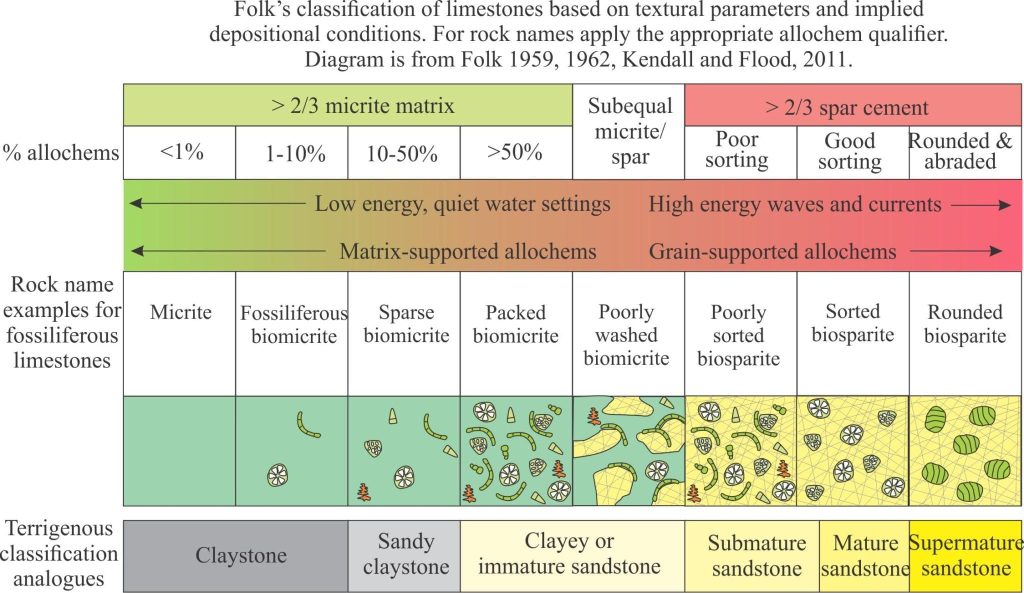
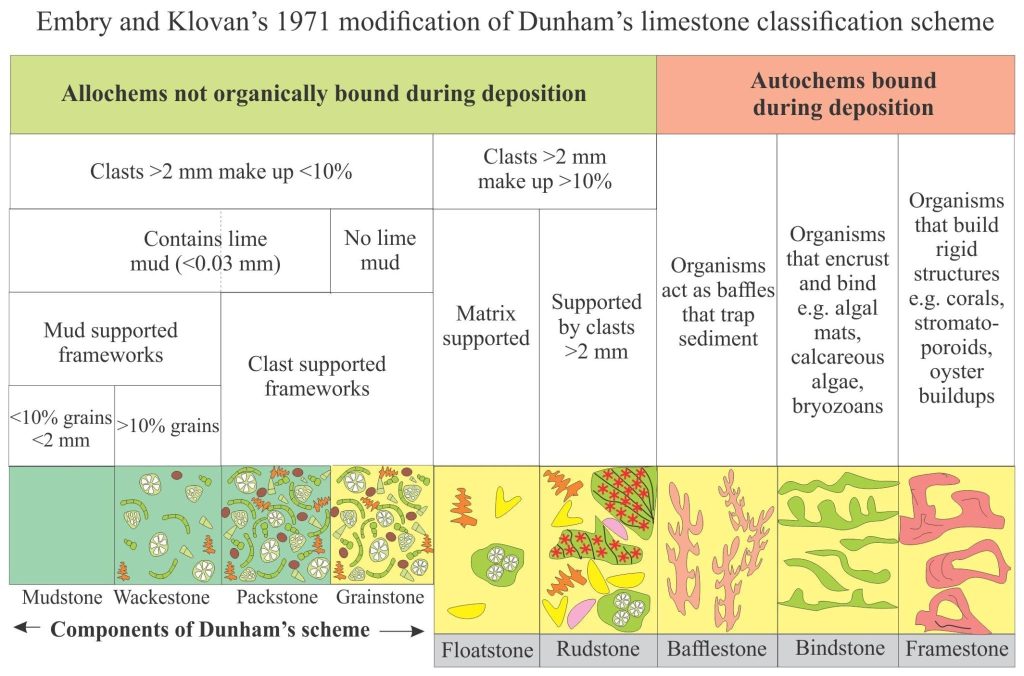
Mineralogy of carbonates; classification
Mineralogy of carbonates; carbonate factories
Mineralogy of carbonates; basic geochemistry
Mineralogy of carbonates; cements
Mineralogy of carbonates; sea floor diagenesis
Mineralogy of carbonates; Beachrock
Mineralogy of carbonates; deep sea diagenesis
Mineralogy of carbonates; meteoric hydrogeology
Mineralogy of carbonates; Karst
Mineralogy of carbonates; Burial diagenesis
Mineralogy of carbonates; Neomorphism
Mineralogy of carbonates; Pressure solution
There is a vast, and for the most part excellent literature on carbonate diagenesis. Here are a few classic and more recent texts that provide much more detail on the subject.
Robin G.C. Bathurst, 1976. Carbonate Sediments and their Diagenesis. Elsevier, Developments in Sedimentology, 12. 658p. An example of the longevity and utility of one of the best on this topic. Now also as an ebook.
Noel James and Phillip Choquette.1984. Diagenesis 5. Limestones; Introduction and subsequent articles on sea floor, meteoric and burial diagenesis. The Canada Geoscience Series on Carbonate Diagenesis is available from the CGS archive.
Noel James and Brian Jones. 2015. The origin of carbonate sedimentary rocks. American Geophysical Union, Wiley works, 464p.An excellent recent update.
Peter Scholle and Dana Ulmer-Scholle, 2003. A colour guide to the petrography of carbonate rocks: grains, textures, porosity, diagenesis. AAPG Memoir 77. Loaded with images.
R.C. Surdam, L.J. Crossey, E.S. Hagen, & H.P. Heasler. 1989. Organic-inorganic interactions and sandstone diagenesis. AAPG, v.73, p. 1-23.
SEPM Strata. Diagenesis and porosity. Part of SEPM’s online stratigraphic web contructed originally by Christopher Kendall. An excellent resource for pretty well anything sedimentological and stratigraphic. Continually updated.
Erik Flugel. 2010. Microfacies of carbonate rocks: Analysis, interpretation and application. Springer. The ebook is cheaper